

They will then share their kinetic energy with the orbital electrons, causing them to be promoted. Or it can be done by bombarding the atoms with electrons. Therefore constantly absorbing/emitting photons from the electromagnetic They only remain in an excited state for less than a microsecond and are In electromagnetic energy of a wide continuous spectrum (usually of visible light - but it is the same principle for other sections of the electromagnetic spectrum - only then you wouldn't 'see' the result - you would need to use a detector).Ībsorb the photons they need to make transitions to higher energy levelsĪnd then give them back out again when they return to the ground state. This can be done by bathing the gas sample Radiation (linked to its wavelength by c = f ) ΔE = hf = (in joule) [To convert to eV divide by 1.6 x 10 -19į = frequency of the photon of electromagnetic Or emitted -the energy interval between the energy levels
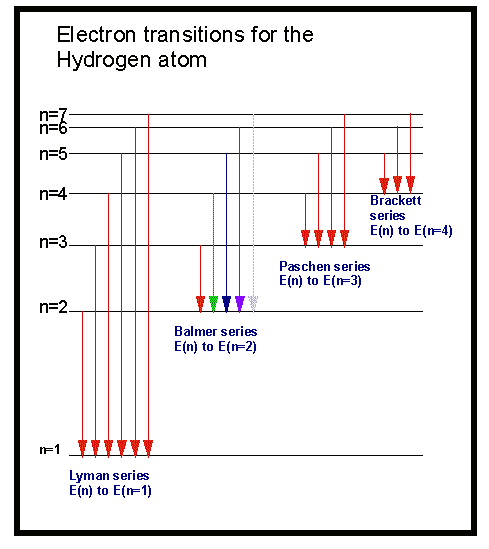
When absorbing or emitting a photon, the size of that photon'sĮnergy depends on the energy gap between the levels. W = QV, so an electron, having a charge 'e' will obtain energy of XeV if placed in an potential difference of X volts.
#Atomic spectra light energy and electron structure free#
It will take just the amount of energy it needs for the jump from the free electron's kinetic energy.Īn electron can obtain energy from an electrical supply. Obtaining energy by colliding with a free electron. Photon absorption - absorbing a photon of exactly the correct energy needed for a 'jump'. It can obtain the energy required for 'promotion' to a higher energy state by several means: Of an atom it either absorbs energy for the transition or emits a photon when reducing its potential energy. When an electron jumps between energy levels We can put information about these energy states into a diagram When it is closer to the nucleus it has less potential energy (needsĮnergy input to escape the influence). Nucleus' influence it is said to have zero potential energy - a zero energy The further away from the nucleus these states are, the higher the potentialĮnergy of the electron in that state. Energy states within the atom (called orbitals by chemists).


 0 kommentar(er)
0 kommentar(er)
